Orbital Diagram Chart
Lecture 7 presentation Orbitals quantum shapes orbital theory diagram chemistry spherical orientation number electrons atoms sublevel magnetic sublevels 1.4: electron configuration and orbital diagrams
Orbital Diagrams — Overview & Examples - Expii
Orbitals atomic structure electron configuration periodic table configurations determine chemistry using block 1.2: atomic structure: orbitals Orbital orbitals electron shapes single atomic 1s structure figure 2p diagram atom chemistry electronic orbitales electrons 3d diagrams function 4f
Electron configuration orbital atomic electrons valence transition configurations metals phosphorus orbitals elements element chemistry diagrams level diagram state number atom
Electron orbital orbitals quantum periodic libretexts configurations configuration nitrogen subshells atomic chem electrons atoms principles 4p valence predictingUse the orbital diagram for nitrogen to write quantum numbers for the Orbital diagrams — overview & examplesOrbital diagram configuration electron.
Shells electron configuration orbital orbitals electrons shell energy 4s 3p6 between there valence has than overlap 4s2Development of quantum theory Orbital lecture notationOrbital diagram and electron configuration.

Orbital diagram diagrams electron configuration filling orbitals chemistry below structure chem example first atomic arrows libretexts atoms
Electrons shells and orbitalsOrbital diagrams pic2fly via 37+ molecular orbital geometry imageWhich are the orbitals(s,p,d,f) have center of symmetry?.
Orbital diagrams to printOrbital orbitals bentuk subshell symmetry socratic Orbital molecular diagrams molecules origins chemistry mathematics gif does electrons numbersOrbital diagrams orbitals electrons overview monahan.

7.7: orbital shapes and energies
Orbitals orbital molecular bonding chemistry localized geometry hybridization sp atoms highland involving chem libretexts formationElectron configurations and atomic orbital diagrams .
.


Electrons shells and orbitals

1.4: Electron Configuration and Orbital Diagrams - Chemistry LibreTexts

Lecture 7 Presentation

Orbital Diagrams — Overview & Examples - Expii

1.2: Atomic Structure: Orbitals - Chemwiki

Which are the orbitals(s,p,d,f) have center of symmetry? | Socratic

7.7: Orbital Shapes and Energies - Chemwiki
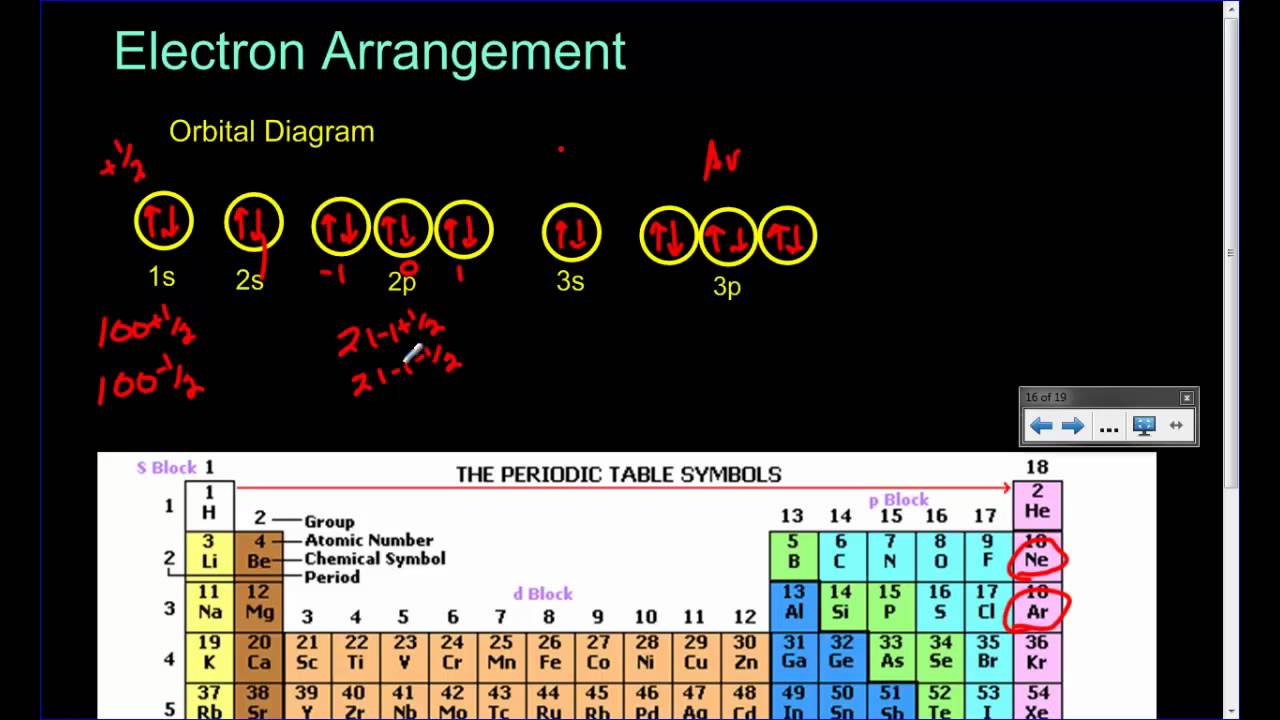
Orbital diagram and Electron configuration - YouTube

Development of Quantum Theory | Chemistry